Methofill® is indicated for the treatment of active rheumatoid arthritis in adult patients, polyarthritic forms of severe, active juvenile idiopathic arthritis when the response to nonsteroidal anti-inflammatory drugs (NSAIDs) has been inadequate, severe recalcitrant disabling psoriasis which is not adequately responsive to other forms of therapy such as phototherapy, psoralen plus ultraviolet A (PUVA), and retinoids, and severe psoriatic arthritis in adult patients and mild to moderate Crohn’s disease either alone or in combination with corticosteroids, in adult patients refractory or intolerant to thiopurines.
With Methofill® SelfDose pre-filled injector you can help your patients get to grips with their methotrexate injections
Methofill® SelfDose pre-filled injector has been designed with a range of features specifically to help your methotrexate patients.1,2
- Created with an ergonomic handle, designed for ease of control3
- 50mg/ml concentration. Low subcutaneous injection volumes are associated with reduced injection site pain4–6
- Patients can control the speed of the injection
- Simply push down to deliver the injection, no need to reach for a button
- The passive needle guard is automatically activated following a successful injection to protect patients from needle-stick injuries7
- An audible ‘click’ and visible yellow band reassures patients they have successfully administered their full injection
- A broad base to place on the skin, with no need to pinch the skin before injection

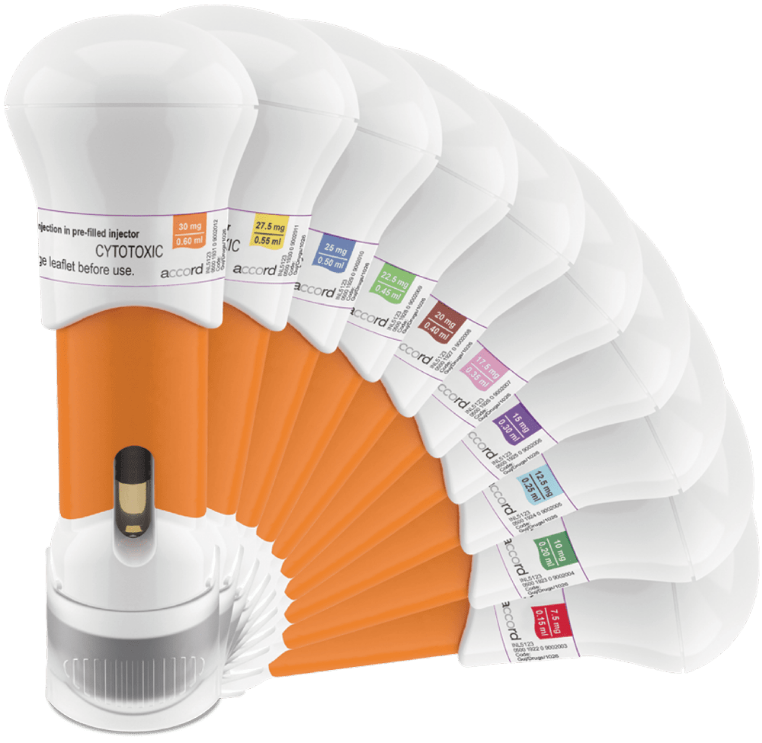
Methofill® SelfDose pre-filled injector is available in 10 colour-coded doses (7.5mg–30mg), which could aid precise and personalised prescribing and help prevent dispensing errors.
Methofill® is also available as a Pre‑Filled Syringe (PFS)
Methofill® SelfDose pre-filled injector has demonstrated results in a real-world setting
In an Accord-funded survey study conducted between April 2019 and May 2020 that gathered experience from 63 adult patients using Methofill® SelfDose pre-filled injector, results demonstrated that little things really can make the difference:8
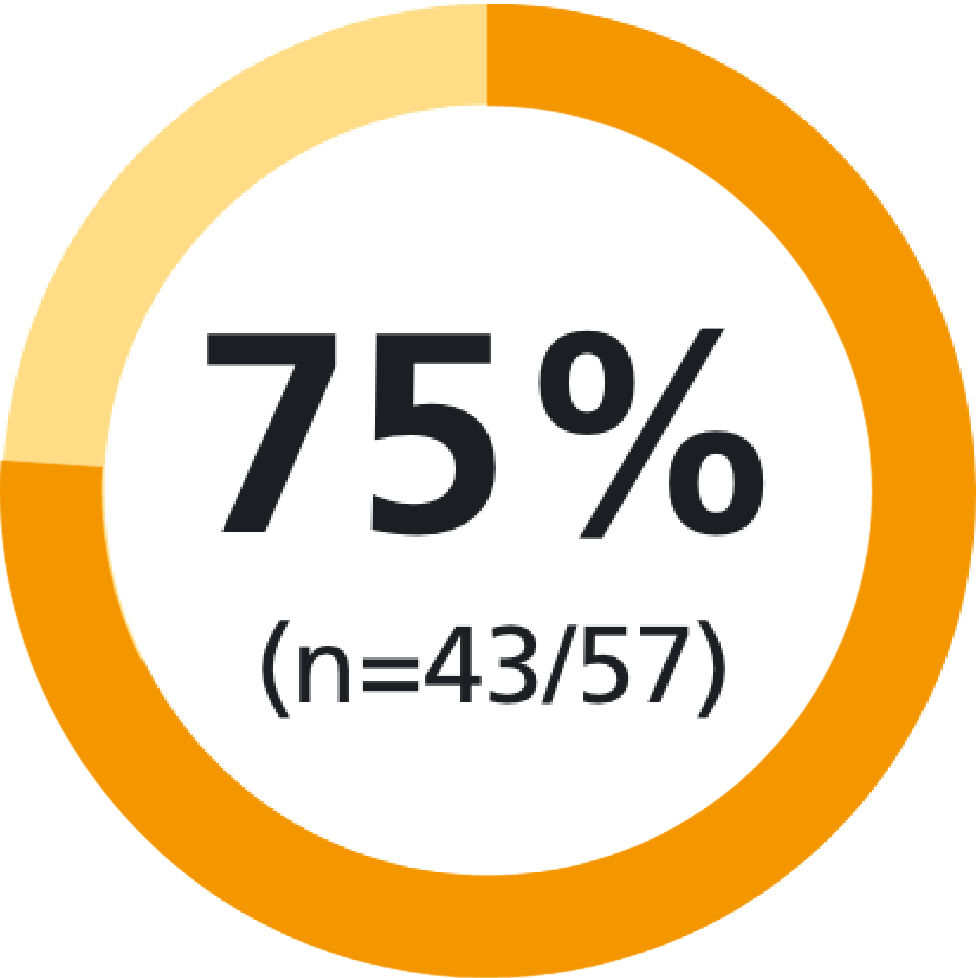
of patients found Methofill® SelfDose pre-filled injector easy to grip9
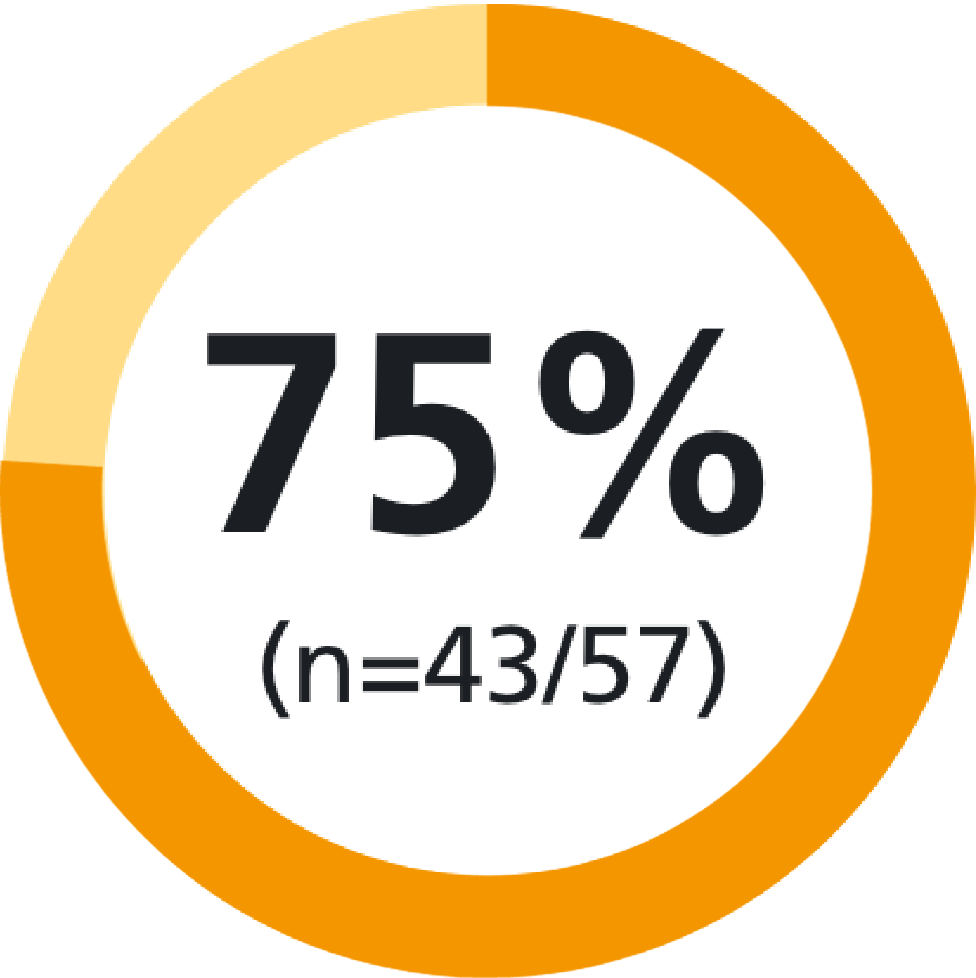
of patients thought the device was easy to use10
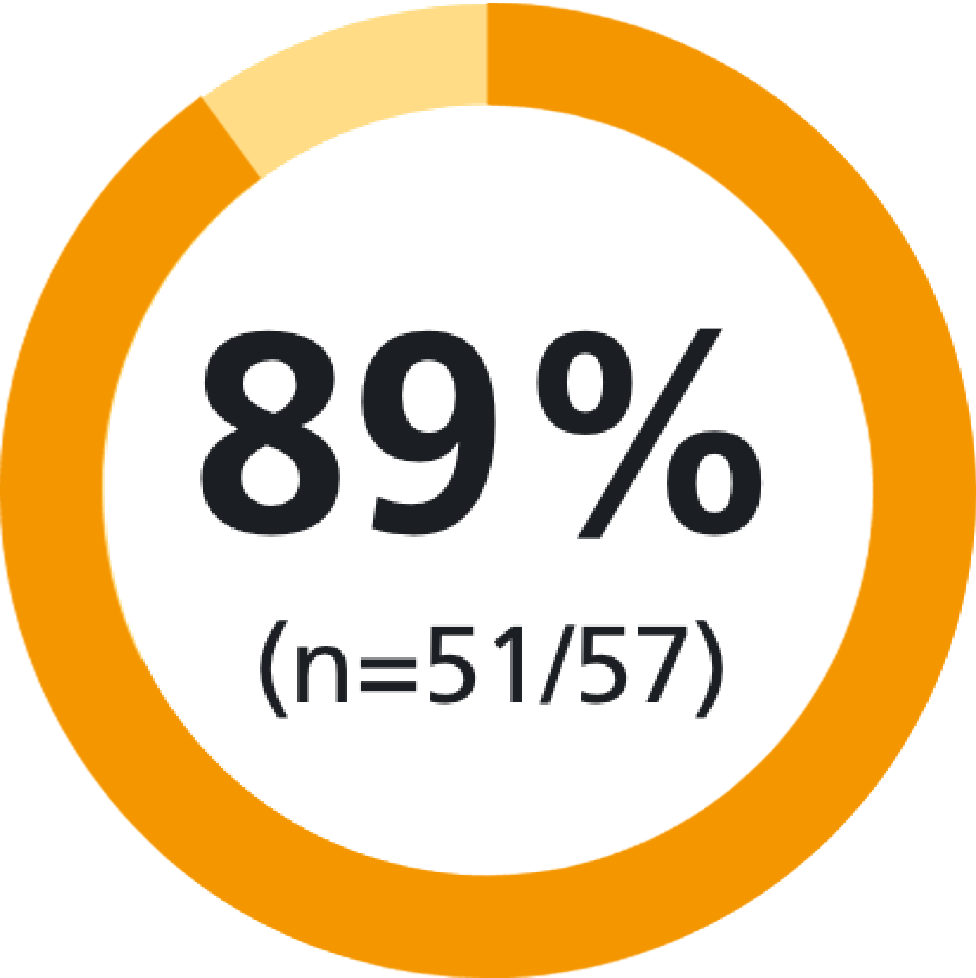
of patients were confident to inject using Methofill® SelfDose pre-filled injector11
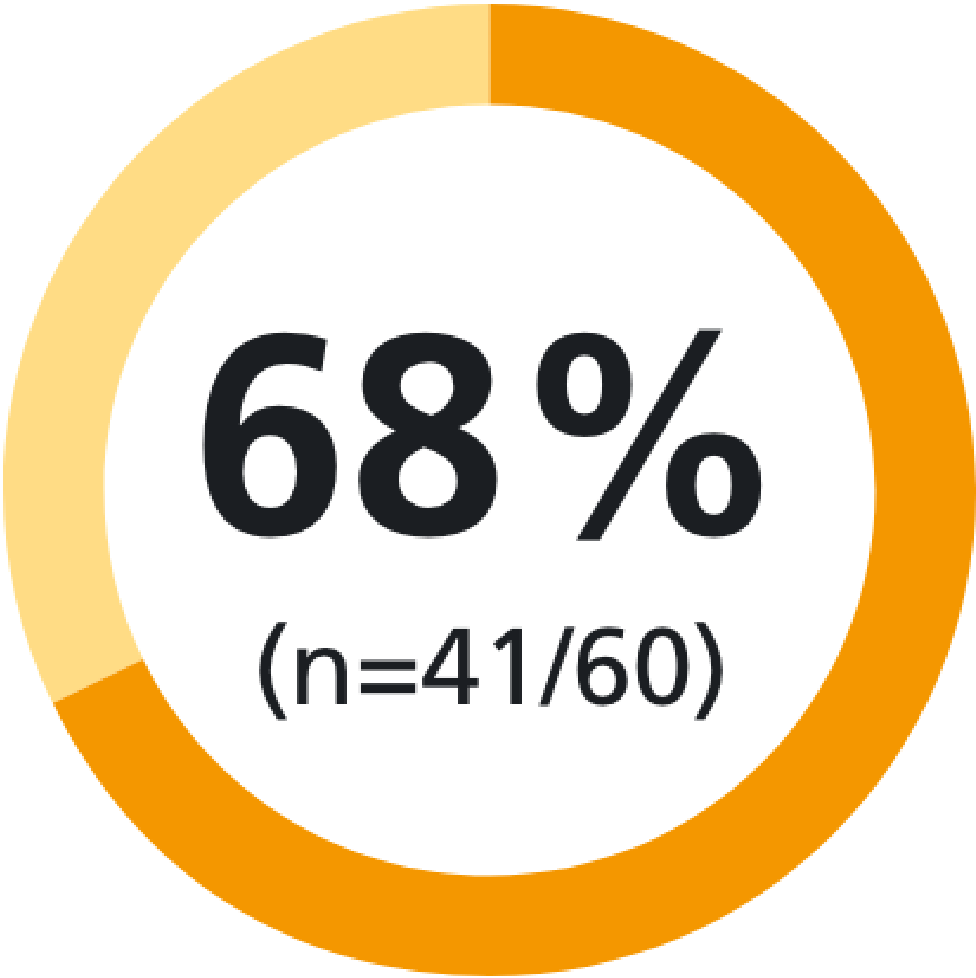
of patients were satisfied* with Methofill® SelfDose pre-filled injector12
*Satisfaction defined as a score of ≥7 on a scale of 1–10.12This demonstrated, in a real-world setting, that the simplicity of the Methofill® SelfDose pre-filled injector helps to put patients in control and feel confident in self-injecting.8
- Patients were diagnosed with either active rheumatoid arthritis, psoriasis vulgaris, psoriatic arthritis or Crohn’s disease
- Recruitment by pharmacists at Day Lewis pharmacies across England
- Cross-sectional, self-reported, paper-based questionnaire written by Accord-UK Ltd
- The majority of the patients were 60 or over (62%, n=37) and female (61%, n=35)
- 3 patients didn’t end up using Methofill® and 3 patients had someone else administer
Using Methofill® SelfDose pre-filled injector
The following video will teach you how to your train your patients on using their Methofill® SelfDose pre-filled injector. All patients should receive training on the correct way to use Methofill® SelfDose pre-filled injector before attempting to inject.
Disposing of Methofill® SelfDose pre-filled injector
For ease of disposal, Methofill® SelfDose pre-filled injector requires a cytotoxic sharps bin with a large aperture and fill volume. The Sharpsafe® and Sharpak® sharps bin brands provide a range of volumes that the Methofill® SelfDose pre-filled injector comfortably fits into.

| Appropriate Sharpsafe® cytotoxic sharps bins:13 | ||
|---|---|---|
| England & Wales | England & Wales & Scotland | |
| Size | NHS Supply chain code | Product code |
| 1.8L | N/A | 50502420 |
| 2L | FSL2299 | 51405420 |
| 5L | FNC85334 | 51025420 |

| Appropriate Sharpak® cytotoxic sharps bins:14,15 | ||
|---|---|---|
| England & Wales | England & Wales & Scotland | |
| Size | NHS Supply chain code | Product code |
| 6L | FSL018 | SHK/CA1526VSP |
| 12L | FSL020 | SHK/CA1536VSP |
| 22L | FSL021 | SHK/CA1548VSP |

Resources
Accord provide a range of support services to support you and your patients throughout their treatment journey.
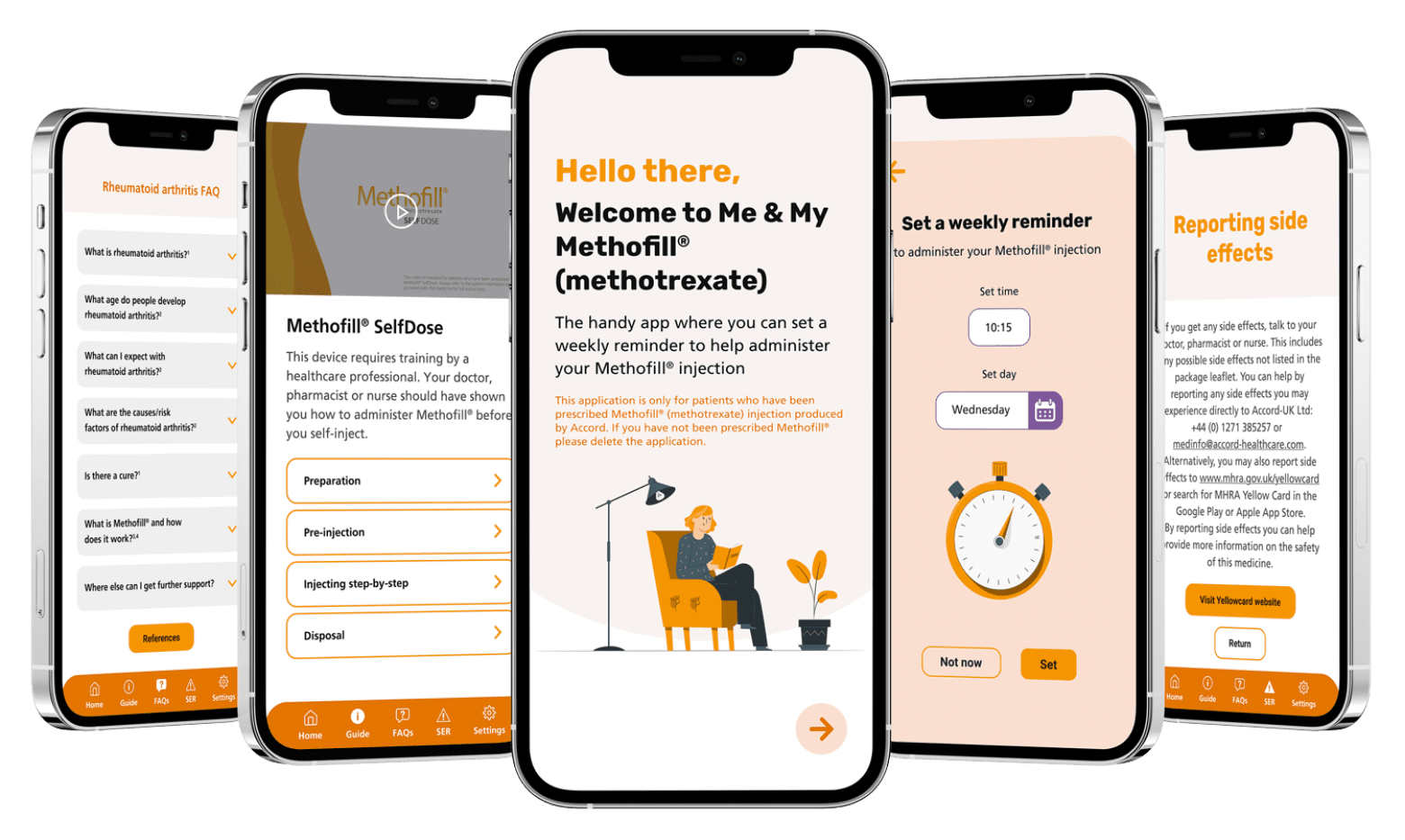
Introducing the Me and My Methofill® app
The Me and My Methofill® app has been developed for your patients who have been prescribed Methofill®. The app contains features to support your patients with their self-injection.
Other HCP resources

Methofill® SelfDose pre‑filled injector HCP trainer guide
This leaflet aids you with patient training on the Methofill® SelfDose pre-filled injector. All patients should receive training on the correct way to use Methofill® SelfDose pre-filled injector before attempting to inject.

Methofill® SelfDose pre‑filled injector overview leavecard
This document provides an overview of the Methofill® SelfDose pre-filled injector and how it could benefit you and your patients.

Methofill® SelfDose pre‑filled injector Psoriasis overview leavecard
This document provides an overview of the potential benefits of Methofill® SelfDose pre-filled injector for your Psoriasis patients.

Methofill® SelfDose pre‑filled injector Crohn's overview leavecard
This document provides an overview of the potential benefits of Methofill® SelfDose pre-filled injector for your Crohn’s patients.

Methofill® SelfDose pre-filled injector Juvenile Idiopathic Arthritis overview leavecard
This document provides an overview of the potential benefits of Methofill® SelfDose pre-filled injector for your Juvenile Idiopathic Arthritis patients.
Other patient resources
These materials are patient resources and are not to be shown to patients from this website.
Methofill® SelfDose pre-filled injector patient guide
Following training with a healthcare professional, the Methofill® SelfDose pre-filled injector guide for patients provides information that they need in order to help them successfully perform their self-injections.
Request the above resources by emailing
enquiries_specialitybrands@accord-healthcare.com

Hear from the experts and find out more about best practices in the treatment of autoimmune patients.
The online platform hosts a range of on-demand videos. Each video is created and presented by experts in their field and covers a different topic, such as the treatment of patients in the remote and rural setting.
Visit the site to see what new content has been added.

References
1. Methofill® solution for injection in pre-filled injector. SmPC. 2. Methofill. Package leaflet: Information for the user. 3. Data on file UK-01467. 4. Müller-Ladner U, et al. Open Rheumatol J. 2010;4:15–22. 5. Jørgensen JT, et al. Ann Pharmacother. 1996;30:729–732. 6. Heise T, et al. Diabetes Obes Metab. 2014;16:971–976. 7. Data on file UK-01464. 8. Methofill® Patient Survey Report REF-03864. 9. Data on file UK-01931. 10. Data on file UK-01943. 11. Data on file UK-01944. 12. Data on file UK-01945. 13. Vernacare. Sharpsafe website. Available from: www.sharpsafe.co.uk/products/ (Last accessed March 2025). 14. Inpress Plastics. Sharpak website. Colour Code Range UK. Available from: https://sharpaks.com/wp-content/uploads/2022/03/COLOUR_CODE_RANGE_INP.pdf (Last accessed March 2025). 15. Inpress Plastics. Sharpak website. Available from: https://sharpaks.com/product/hydri-purple-range/ (Last accessed March 2025).
Methofill® (methotrexate) SelfDose pre-filled injector Summary of Product Characteristics (SmPC), legal category, cost and adverse event reporting:
Legal Category POM: Prescription only medicine
Methofill 7.5mg solution for injection in a pre-filled injector NHS list price £12.86
Methofill 10mg solution for injection in a pre-filled injector NHS list price £13.25
Methofill 12.5mg solution for injection in a pre-filled injector NHS list price £14.34
Methofill 15mg solution for injection in a pre-filled injector NHS list price £14.40
Methofill 17.5mg solution for injection in a pre-filled injector NHS list price £15.24
Methofill 20mg solution for injection in a pre-filled injector NHS list price £15.55
Methofill 22.5mg solution for injection in a pre-filled injector NHS list price £16.10
Methofill 25mg solution for injection in a pre-filled injector NHS list price £16.12
Methofill 27.5mg solution for injection in a pre-filled injector NHS list price £16.49
Methofill 30mg solution for injection in a pre-filled injector NHS list price £16.55
Important warning about the dosage of Methofill® (methotrexate):
Methofill® (methotrexate) must only be used once a week for the treatment of Rheumatoid arthritis, Juvenile arthritis, Psoriatic arthritis, Psoriasis, Crohn’s disease. Dosage errors in the use of Methofill® (methotrexate) can result in serious adverse reactions, including death. Please read the posology section of the summary of product characteristics very carefully.
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk. Adverse events should also be reported to Accord-UK LTD on 01271 385257 or email medinfo@accord-healthcare.com.
These links will open in a new window. Accord is not responsible for content on external websites.